Are You a Healthcare Professional?
This European website, initiated and developed by CSL Behring, has two separate sections with the aim to provide information on haemophilia for an international audience, either to European healthcare professionals or to the general public.*
Yes, I am a healthcare professional*
No, I am not a healthcare professional
Haemophilia and Gene Therapeutics
Haemophilia: an optimal disease to be treated with gene therapy.
Haemophilia Gene Therapy
Gene Therapies That Have Been Tailored for Haemophilia
Patients with haemophilia have a functionally deleterious mutation to their factor VIII (haemophilia A) or IX (haemophilia B) gene which causes the depleted production of functional clotting factors or the production of faulty clotting factors leaving patients vulnerable to bleeding events.1
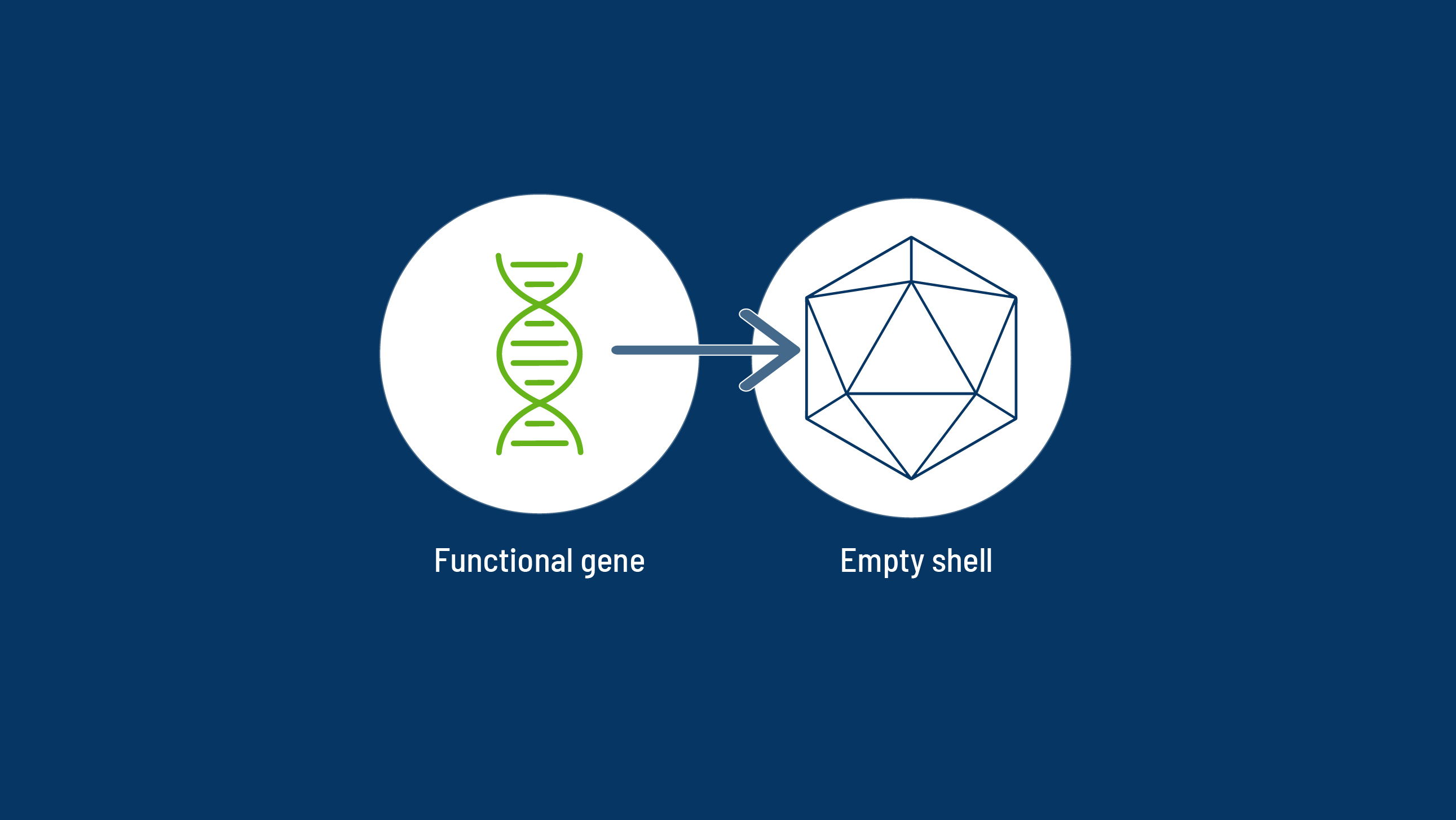
After decades of research, scientists now understand that haemophilia is caused by mutations to a single, monogenic gene. This fact makes haemophilia an optimal target for gene therapy,1 in which viral vectors can be used to deliver a therapeutic transgene.1,2
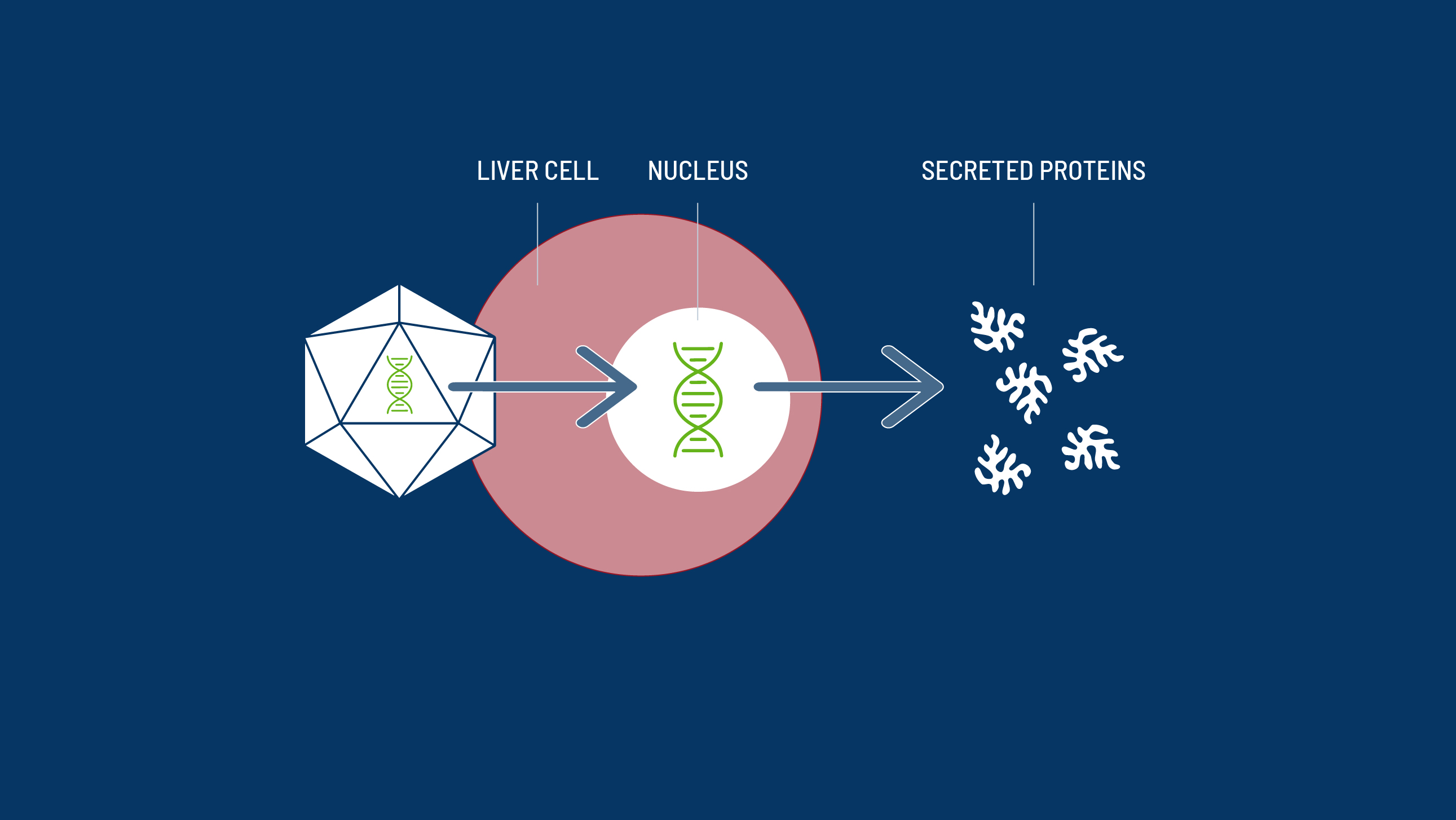
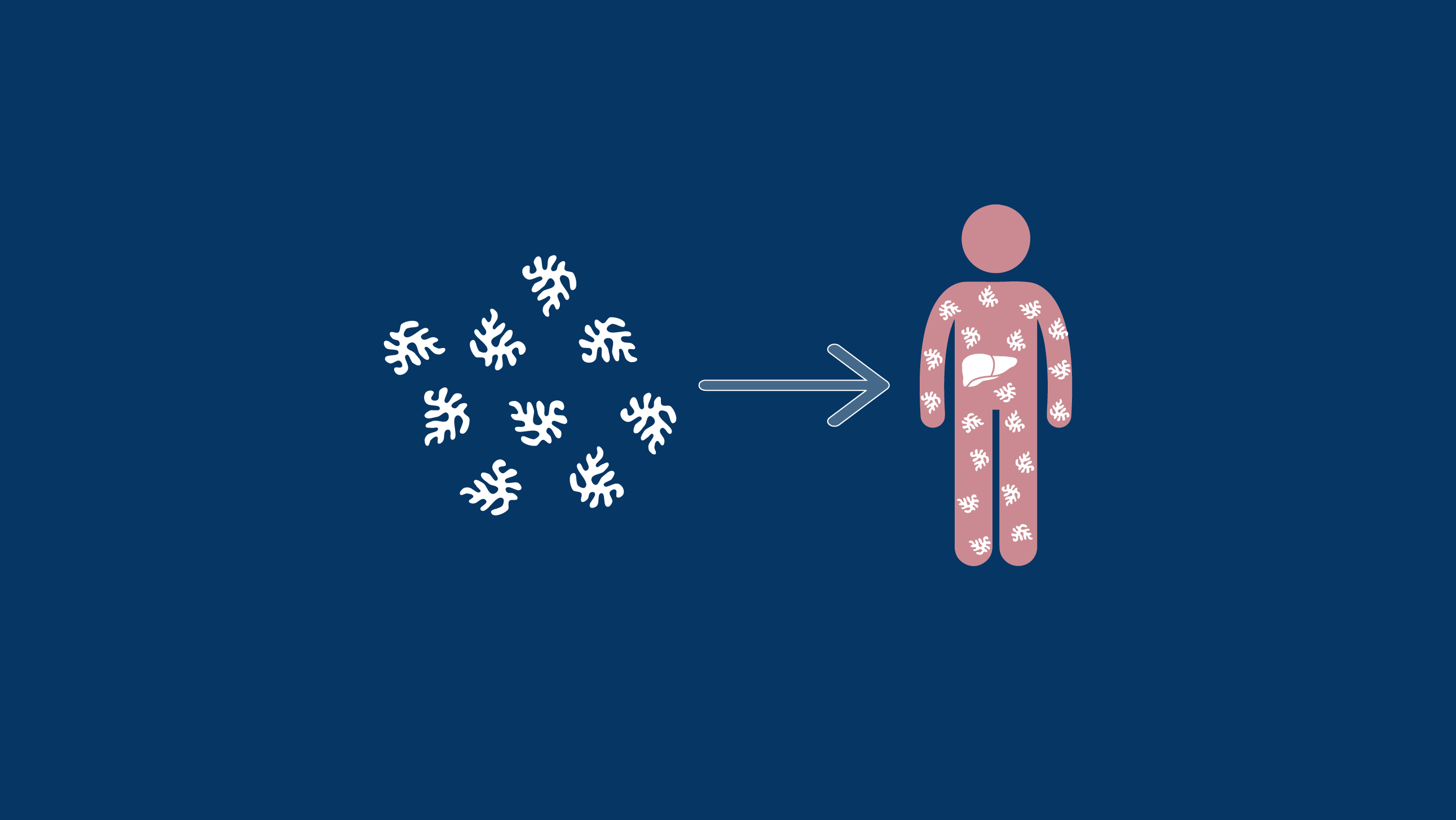
The Mechanism of Action of a Haemophilia-Specific Gene Therapy
All gene therapies have five key steps from vector engineering to drug delivery.
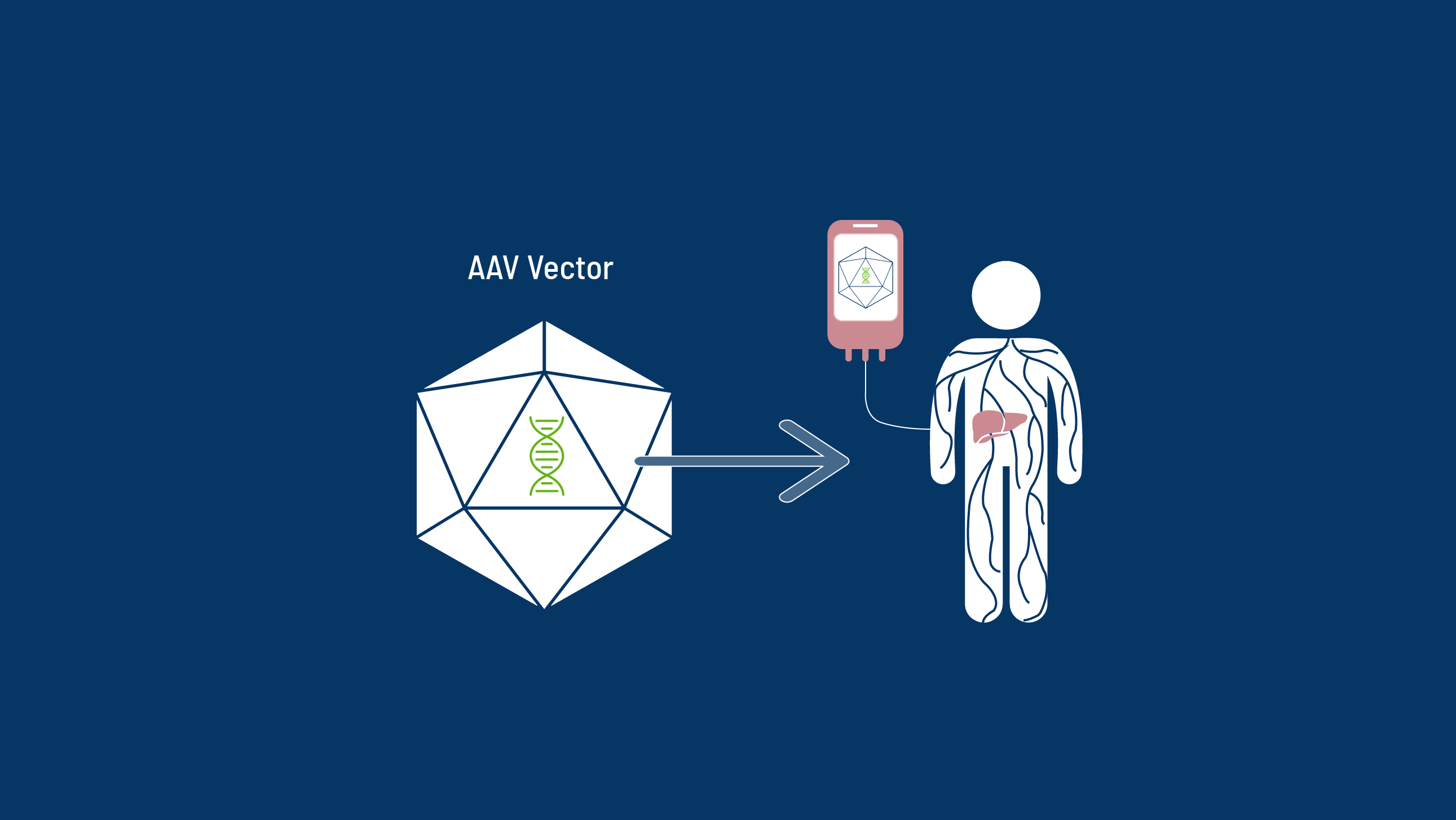
The Role of AAV
AAV-Vector Types Explored in Haemophilia
The clinical use of AAV-based gene therapy for haemophilia has been investigated for almost two decades8,9 with the goal of both improving disease management and releasing patients from the relentless treatment schedule.5,7,9
AAV vector gene therapies convert a natural virus into a beneficial form. There are many AAV serotypes which infect humans. Each of these serotypes has differing features that scientists can leverage in order to tailor a gene therapy for a certain disease.10 Some of the more commonly used serotypes for gene therapy include:
AAV2
Early research into AAVs featured this serotype and it was the first AAV to be successfully cloned and sequenced, establishing the fundamental knowledge about the potential use of AAVs in gene therapy.6 This serotype is able to target a broad range of tissues that have heparan sulphate, fibroblast growth factor, hepatocyte growth factor, laminin and a5b1 receptors on their surface.3 However, this serotype is the most vulnerable to host AAV antibodies, as 60%-70% of people have antibodies against this serotype, increasing risk for host immune response.4
AAV5
AAV8
AAV8 can target the liver, skeletal muscle, heart and pancreas by binding to laminin receptors on their surface.3,11
Haemophilia: The OPTIMAL Gene Therapy Target
Haemophilia is the OPTIMAL CANDIDATE for Gene Therapy
Haemophilia is a monogenic, X-linked inherited disease. Functional deleterious mutations to the factor F8 or F9 genes cause a depletion of functional clotting factors, leaving patients vulnerable to dangerous bleeding events. Treatment for this disease is life-long and burdensome physically, mentally and financially.1,12
Although haemophilia is undoubtably a debilitating disease, the good news is that these features make haemophilia an optimal target for gene therapy.1
What Makes Haemophilia Fit for Gene Therapy?
The goal of gene therapy is to maintain durable expression of a therapeutic gene in order to ameliorate symptoms or even cure a disease.2 Unfortunately, not all diseases are candidates for this type of therapy. Compatibility with gene therapies relies on several factors, including:
Frequently Asked Questions
Previous
Gene Therapy
Next
Preparing for Gene Therapy
References
- Doshi BS, Arruda VR. Gene therapy for hemophilia: what does the future hold? Ther Adv Hematol. 2018;9(9):273-293.
- High KA, Roncarolo MG. Gene therapy. N Engl J Med. 2019;381(5):455-464.
- Naso MF, Tomkowicz B, Perry WL III, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31:317-334.
- Tseng Y-S, Agbandje-McKenna M. Mapping the AAV capsid host antibody response toward the development of second generation gene delivery vectors. Frontiers Immunol. 2014;5(9):1-11.
- Nathwani AC, Reiss UM, Tuddenham EGD, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994-2004.
- Wang D, Tai PW, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18(5):358-378.
- Rangarajan S, Walsh L, Lester W, et al. AAV5-Factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377(26):2519-2530.
- Arruda V, Stedman H, Jian H, et al. Correction of hemophilia B phenotype by novel method of regional intravenous delivery of AAV vector to skeletal muscle of hemophilia B dogs. Mol Ther. 2005;11:S233.
- Leebeek FWG, Miesbach W. Gene therapy for hemophilia: a review on clinical benefit, limitations, and remaining issues. Blood. 2021;138(11):923-931
- Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Micro Rev. 2008;583-593.
- Pipe S, Leebeek FWG, Ferreira V, Sawyer EK, Pasi J. Clinical considerations for capsid choice in the development of liver-targeted AAV-based gene transfer. Mol Ther Methods Clin Dev. 2019;15:170-178.
- Perrin GQ, Herzog RW, Markusic DM. Update on clinical gene therapy for hemophilia. Blood. 2019;133(5):407-414.
- Arruda VR, Doshi BS. Gene therapy for hemophilia: facts and quandaries in the 21st century. Med J Hematol Infect Dis. 2020;12(1):e2020069.
- Nathwani AC. Gene therapy for hemophilia. Hematology Am Soc Hematol Educ Program. 2019;2019(1):1-8.
- Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011 Dec 22;365(25):2357-65.