Are You a Healthcare Professional?
This European website, initiated and developed by CSL Behring, has two separate sections with the aim to provide information on haemophilia for an international audience, either to European healthcare professionals or to the general public.*
Yes, I am a healthcare professional*
No, I am not a healthcare professional
Haemophilia and Gene Therapeutics
Is Haemophilia a suitable condition to be treated with gene therapy?
Haemophilia Gene Therapy
Gene Therapies That Have Been Tailored for Haemophilia
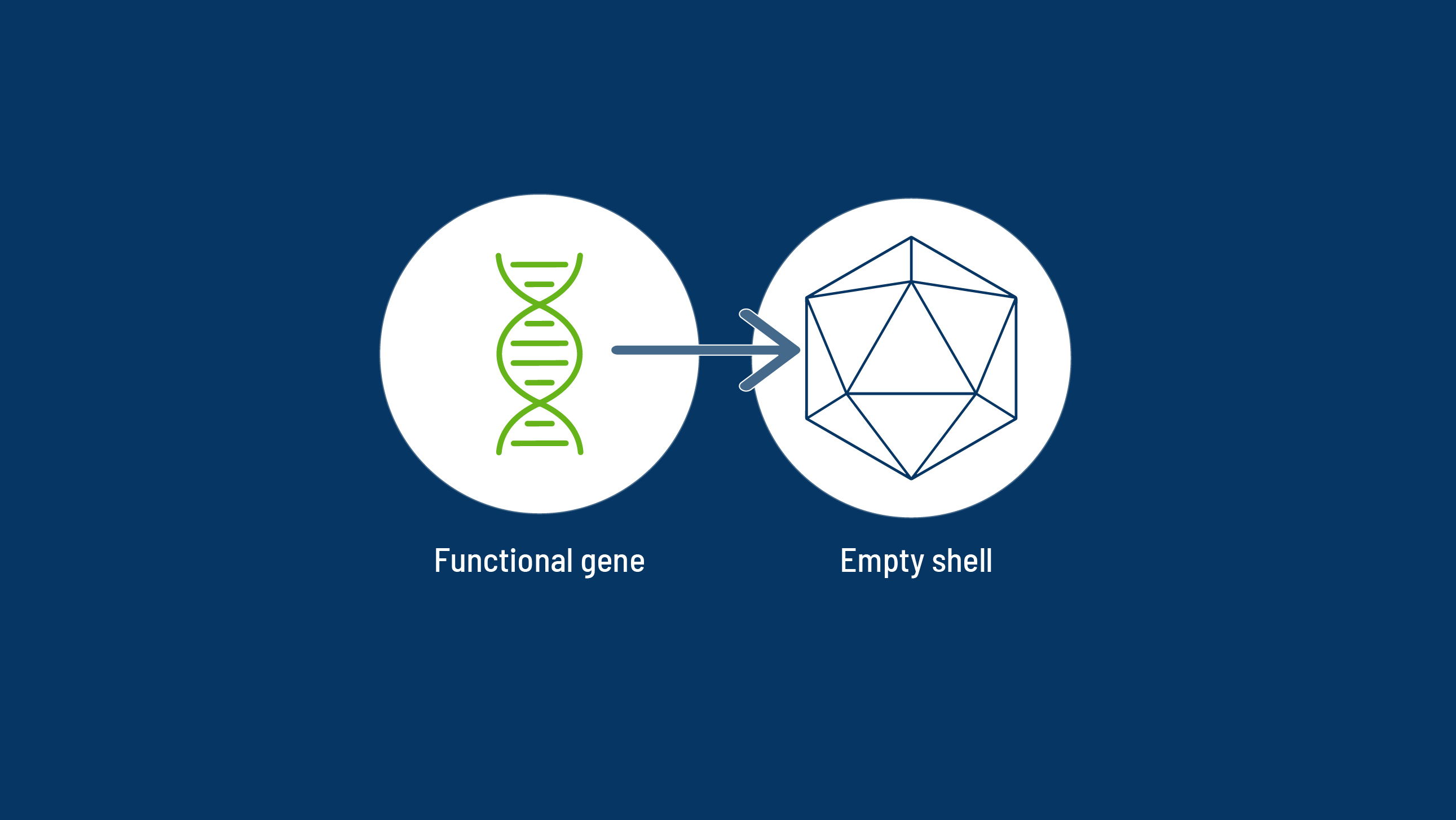
Gene therapy is well suited for genetic diseases that are caused by a single defective gene, like haemophilia.1
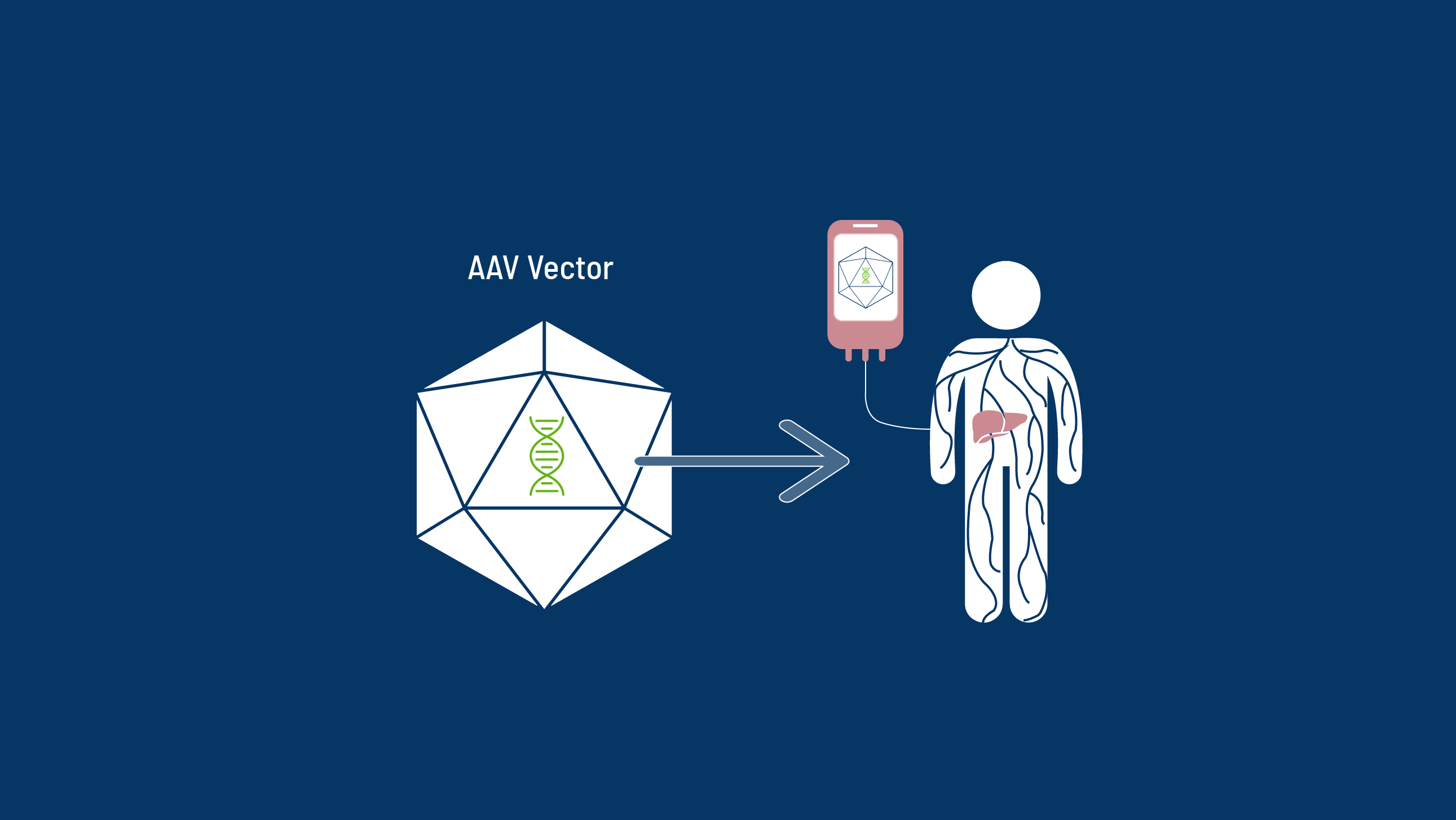
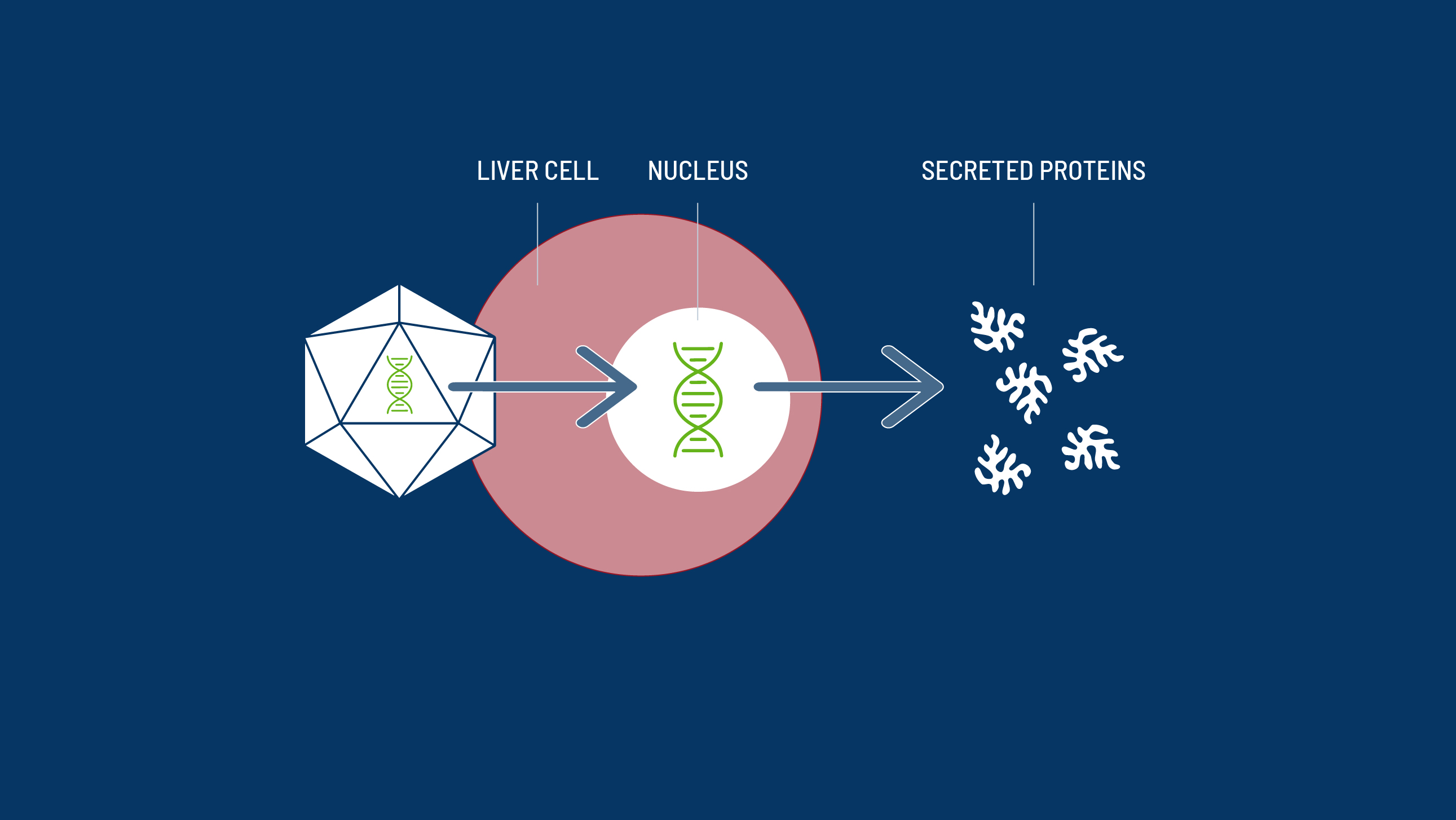
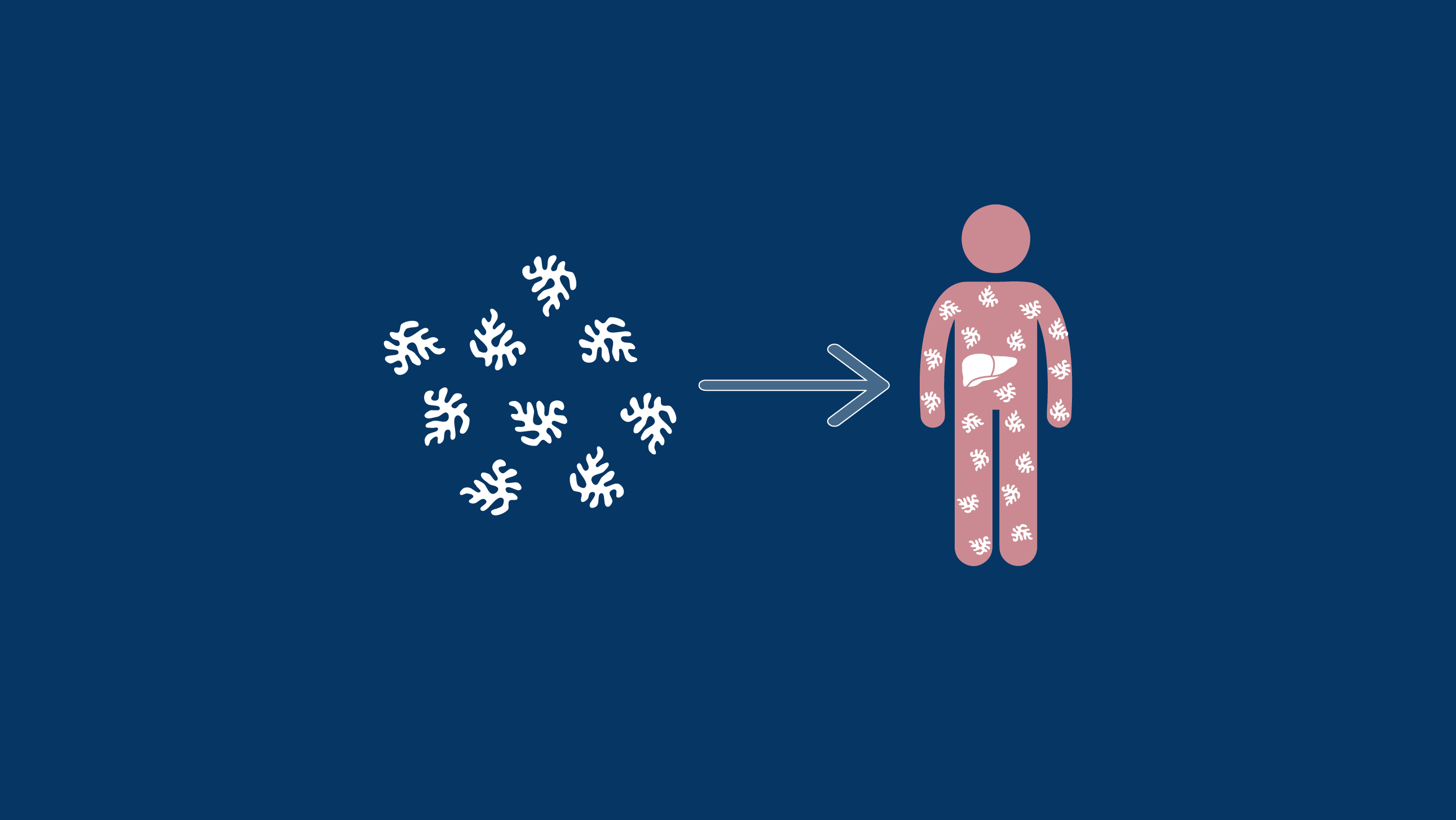
With a one-time gene therapy treatment, it may be possible for someone with haemophilia to generate enough of their own, functional clotting factors to live without a regular infusion schedule.2
Haemophilia: AN OPTIMAL Gene Therapy Target
Is Haemophilia a Good Candidate for Gene Therapy?
Haemophilia occurs because there is a defect to the factor VIII (haemophilia A) or IX (haemophilia B) genes which causes depleted production of factors or non-functional factors to be produced. Without enough clotting factors, people with haemophilia are at risk for uncontrolled bleeding.1 While the characteristics of haemophilia make it a challenging disease to manage and live with, it also makes it an optimal target for gene therapy.7
What Makes Haemophilia Fit for Gene Therapy?
Not all conditions are candidates for gene therapy. Whether or not a certain disease or condition is compatible with this kind of treatment relies on several factors, including:
Frequently Asked Questions
Previous
Gene Therapy
Next
Preparing for Gene Therapy
References
- Doshi BS, Arruda VR. Gene therapy for hemophilia: what does the future hold? Ther Adv Hematol. 2018;9(9):273-293.
- Nathwani AC. Gene therapy for hemophilia. Hematology Am Soc Hematol Educ Program. 2019;2019(1):1-8.
- Heinz S, Braspenning J. Measurement of blood coagulation factor synthesis in cultures of human hepatocytes. Methods Mol Biol. 2015;1250:309-16.
- Naso MF, Tomkowicz B, Perry WL III, Strohl WR. Adeno-associated virus AAV as a vector for gene therapy. BioDrugs. 2017;31:317-334.
- Nathwani AC, Reiss UM, Tuddenham EGD, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994-2004.
- Wang D, Tai PW, Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. 2019;18(5):358-378.
- Perrin GQ, Herzog RW, Markusic DM. Update on clinical gene therapy for hemophilia. Blood. 2019;133(5):407-414.
- Arruda VR, Doshi BS. Gene therapy for hemophilia: facts and quandaries in the 21st century. Med J Hematol Infect Dis. 2020;12(1):e2020069.
- Arruda V, Stedman H, Jian H, et al. Correction of hemophilia B phenotype by novel method of regional intravenous delivery of AAV vector to skeletal muscle of hemophilia B dogs. Mol Ther. 2005;11:S233.
- Nathwani AC, Tuddenham EGD, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. New Engl J Med. 2011;365(25):2357-2365.
- Nathwani AC, Reiss U, Tuddenham EGD, at al. Adeno-associated mediated gene transfer for hemophilia B: 8 year follow up and impact of removing "empty viral particles" on safety and efficacy of gene transfer. Blood 2018;132(Suppl 1):491.
- High KA, Roncarolo MG. Gene therapy. N Engl J Med. 2019;381(5):455-464.
- Food and Drug Administration (FDA) Cellular, Tissue, and Gene Therapies Advisory Committee Meeting #70. Toxicity risks of adeno-associated virus (AAV) vectors for gene therapy (GT). Published September 2021. https://www.fda.gov/media/151969/download Accessed July 14, 2023.